Understanding AEs
Treatments for NHL can cause a variety of adverse events.
Chemotherapy
Common adverse events of chemotherapy include myelosuppression, febrile neutropenic fever, and immunosuppression. Myelosuppression can be treated with transfusions (red blood cells and platelets) or the administration of colony-stimulating factors (eg, granulocyte colony-stimulating factor). Neutropenia leads to an increased risk of infections from bacteria, viruses, and fungi. Patients are also susceptible to infections like varicella-zoster and herpes zoster, which are managed by post-exposure prophylaxis against varicella-zoster infection. Other common adverse events include hair loss, mouth sores, loss of appetite, nausea and vomiting, diarrhea or constipation, increased risk of infection, heart failure, bleeding or bruising after minor cuts or injuries, fatigue, and shortness of breath.1
Radiation Therapy
Radiotherapy can cause heart failure by causing diffuse fibrosis in the interstitium of the myocardium and progressive fibrosis of the pericardial layers, the cells in the conduction system, and the cusps or leaflets of the valves. The left ventricular ejection fraction is usually preserved. Although vincristine is a chemotherapeutic agent, it can cause neurotoxicity. Other adverse events include gastrointestinal symptoms, such as constipation, diarrhea, dry mouth, and mouth sores.1
Long-term survivors of NHL have an elevated risk of developing a second malignancy. The risk of developing a second malignancy varies based on the subtype of NHL and the treatment received. Depending on the area of radiation, patients can develop squamous cell carcinoma of the head and neck, and breast cancer.1
Radiation to the neck and mediastinum can result in hypothyroidism. Patients undergoing hematopoietic cell transplantation with total body irradiation (TBI) conditioning were documented to suffer from growth hormone deficiency, hypogonadism, insulin resistance, and dyslipidemia.1
Stem Cell Transplantation
Hematopoietic cell transplantation can lead to neurologic and psychiatric complications, like post-traumatic stress disorder.1
Immunotherapy
CAR T-cell therapy: Several clinical studies indicate that CAR T cell therapy can cause serious adverse events (SAEs) in patients with NHL (Figure 1). The SAEs can affect any organ system of the body and can develop into multiple organ failure in severe cases, endangering life.2
Bispecific Antibody Therapy
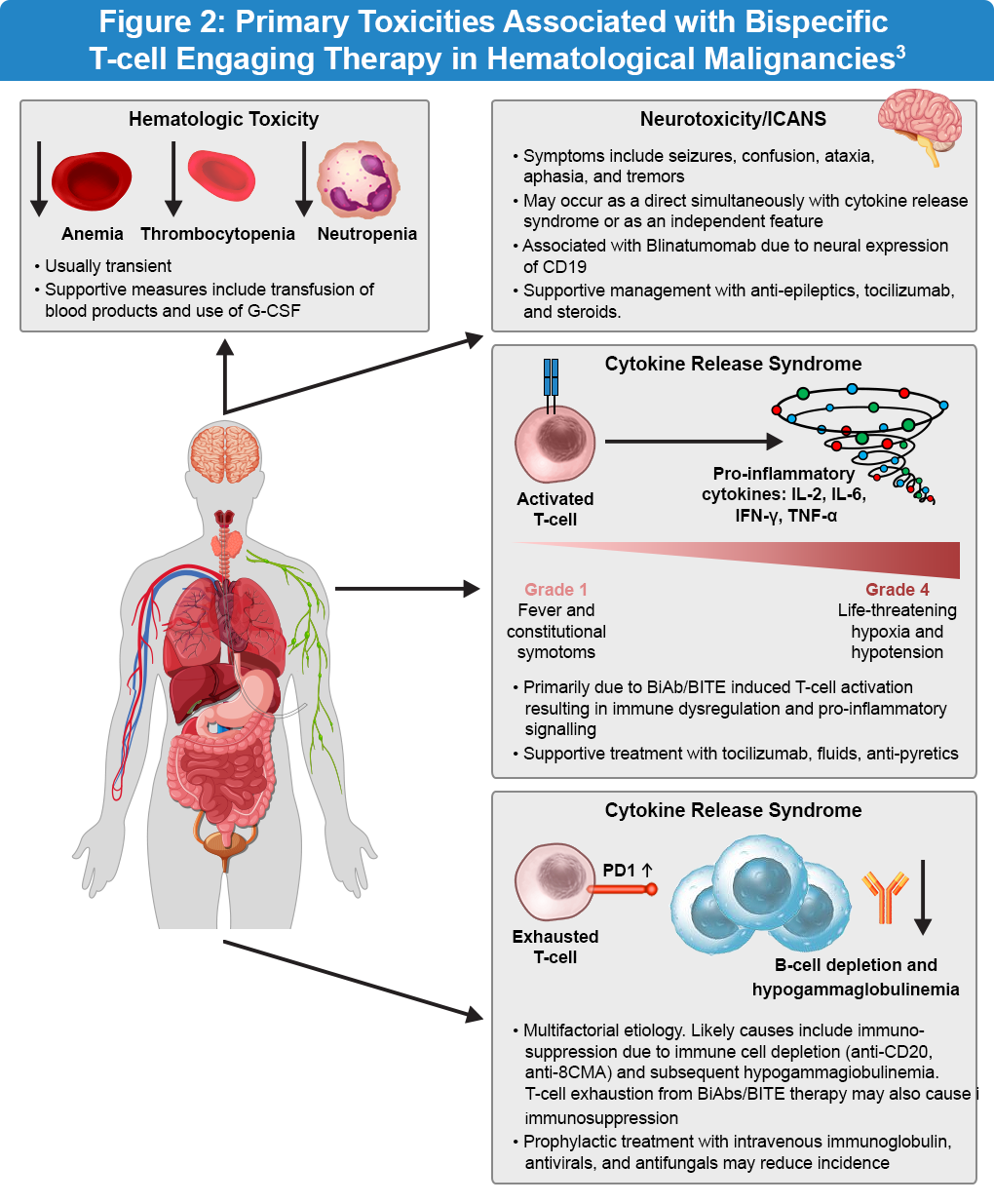
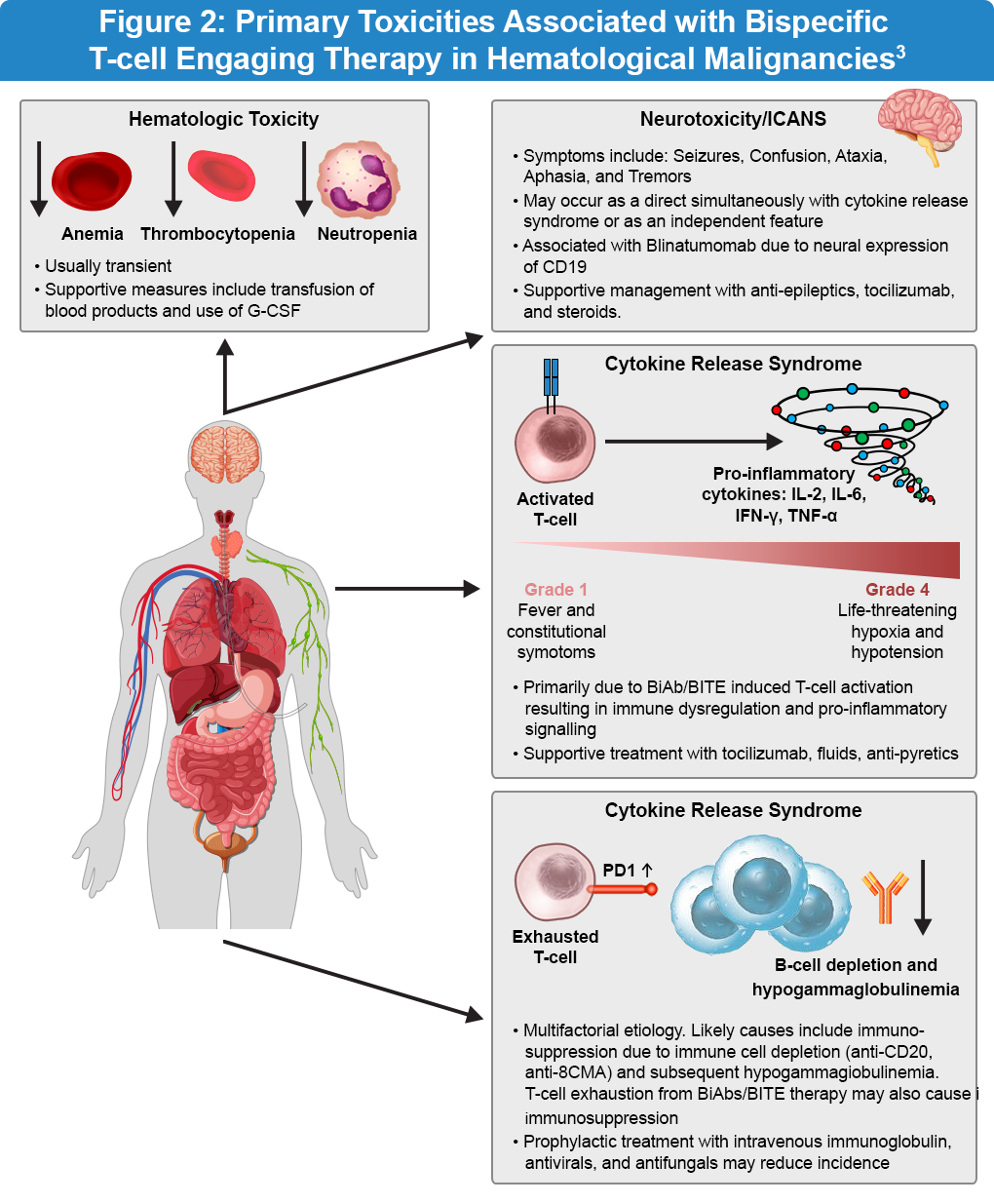
The key adverse events associated with bispecific antibody therapy are cytokine release syndrome (CRS), neurotoxicity, infections, and tumor lysis syndrome (Figure 2). CRS is characterized by an exaggerated inflammatory response with elevated levels of cytokines, including interleukin-2 (IL-2), interleukin-6 (IL-6), interferon-gamma (IFN-γ), and tumor necrosis factor-alpha (TNF-α). The clinical symptoms of CRS range from mild fever and malaise to severe hypotension and hypoxia. In the ELM-1 and ELM-2 clinical studies, a modified step-up dose regimen demonstrated a favorable safety profile. The safety profile was generally consistent with earlier reports and CRS events were predominantly low grade and manageable. Importantly, continued treatment with odronextamab showed no detrimental effects on patient-reported outcomes.4
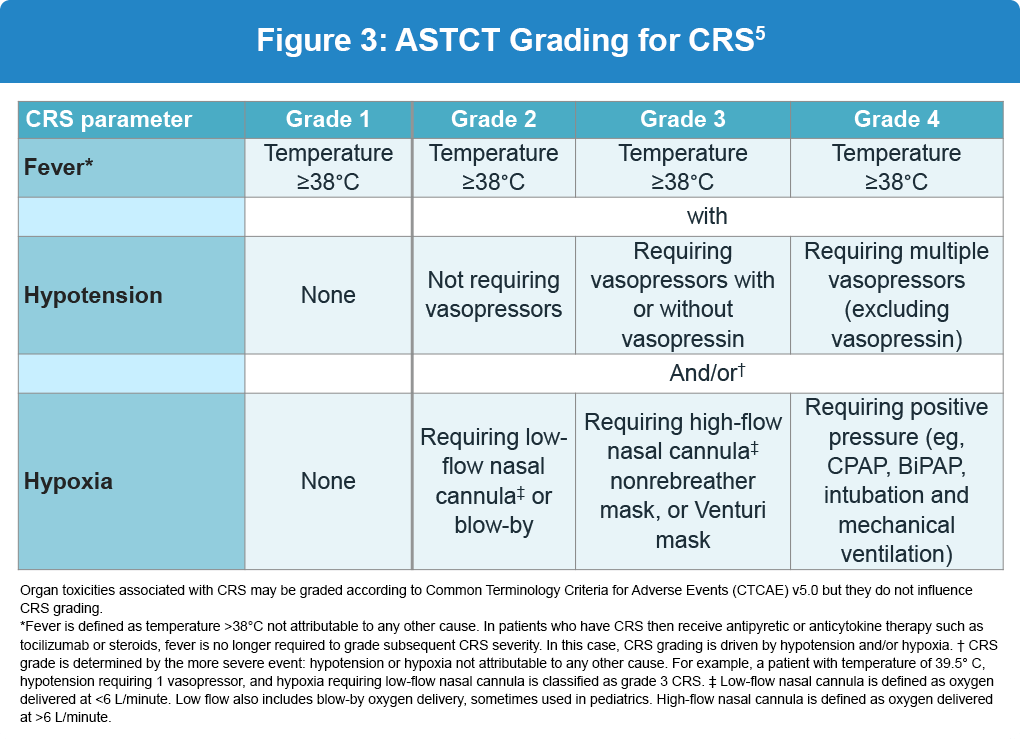
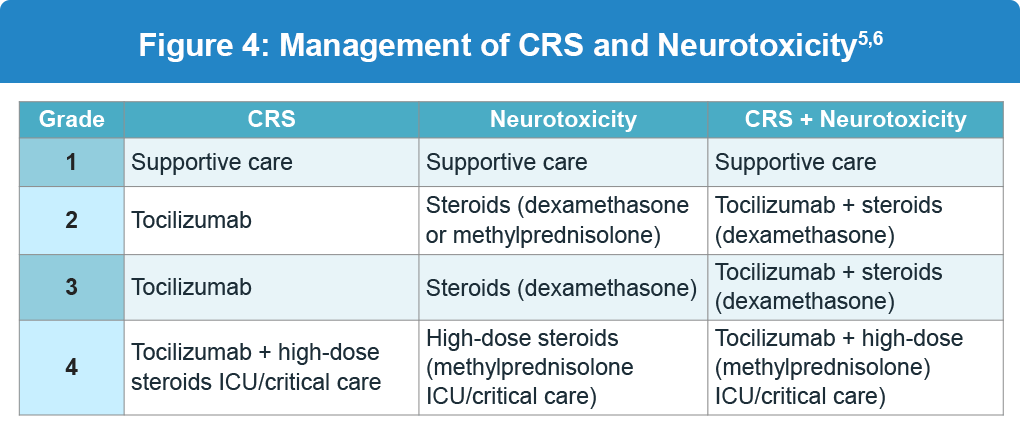
The severity of CRS is graded according to guidelines from the American Society for Transplantation and Cellular Therapy (ASTCT). Grades 1 and 2 are more common and characterized by non-life-threatening symptoms, whereas grades 3 and 4 require urgent intervention due to the life-threatening nature of symptoms (Figure 3). Management of CRS and neurotoxicity based on the grade and is shown below (Figure 4).3,5
- Always rule out/treat alternative causes5,6
- If tocilizumab-refractory, consider corticosteroids5,6
- Patients with neurotoxicity should receive antiepileptic drugs (AEDs) and appropriate CNS imaging, plus EEG monitoring5,6
- Steroid dosing for neurotoxicity may vary between products5,6
- Patients on steroids should receive appropriate fungal prophylaxis5,6
Comprehensive, consensus-based recommendations specific to the assessment and management of bispecific antibody (BsAb)-related toxicities have been developed by an international panel of academic and community practice physicians, advanced practitioners, registered nurses and pharmacists with experience using CD3xCD20 BsAbs.7
References:
- Sapkota S, Shaikh H. Non-Hodgkin Lymphoma. StatPearls. Last updated February 24, 2023. (https://www.ncbi.nlm.nih.gov/books/NBK559328/).
- Chen X, Li P, Tian B, Kang X. Serious adverse events and coping strategies of CAR-T cells in the treatment of malignant tumors. Front Immunol. 2022;13:1079181. doi:10.3389/fimmu.2022.1079181
- Omer MH Shafqat A, Ahmad O, Alkattan K, Yaqinuddin A, Damlaj M. Bispecific antibodies in hematological malignancies: A scoping review. Cancers (Basel). 2023;15:4550. doi:10.3390/cancers15184550
- Taszner M, Luminari S, Cho SG, et al. P1083: Odronextamab in patients with relapsed/refractory follicular lymphoma grade 1-3A: Results from a prespecified analysis of pivotal phase 2 study ELM-2. Hemasphere. 2023;7:e214536e. doi:10.1097/01.HS9.0000971228.21453.6e
- Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019;25:625-638. doi:10.1016/j.bbmt.2018.12.758
- National Comprehensive Cancer Network®. NCCN Clinical Practice Guidelines in Oncology. B-Cell Lymphomas. Version 3.2025. (https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf).
- Crombie JL, Graff T, Falchi L, et al. Consensus recommendations on the management of toxicity associated with CD3xCD20 bispecific antibody therapy. Blood. 2024;143:1565-1575. doi:10.1182/blood.2023022432
ALL URLs accessed October 27, 2025